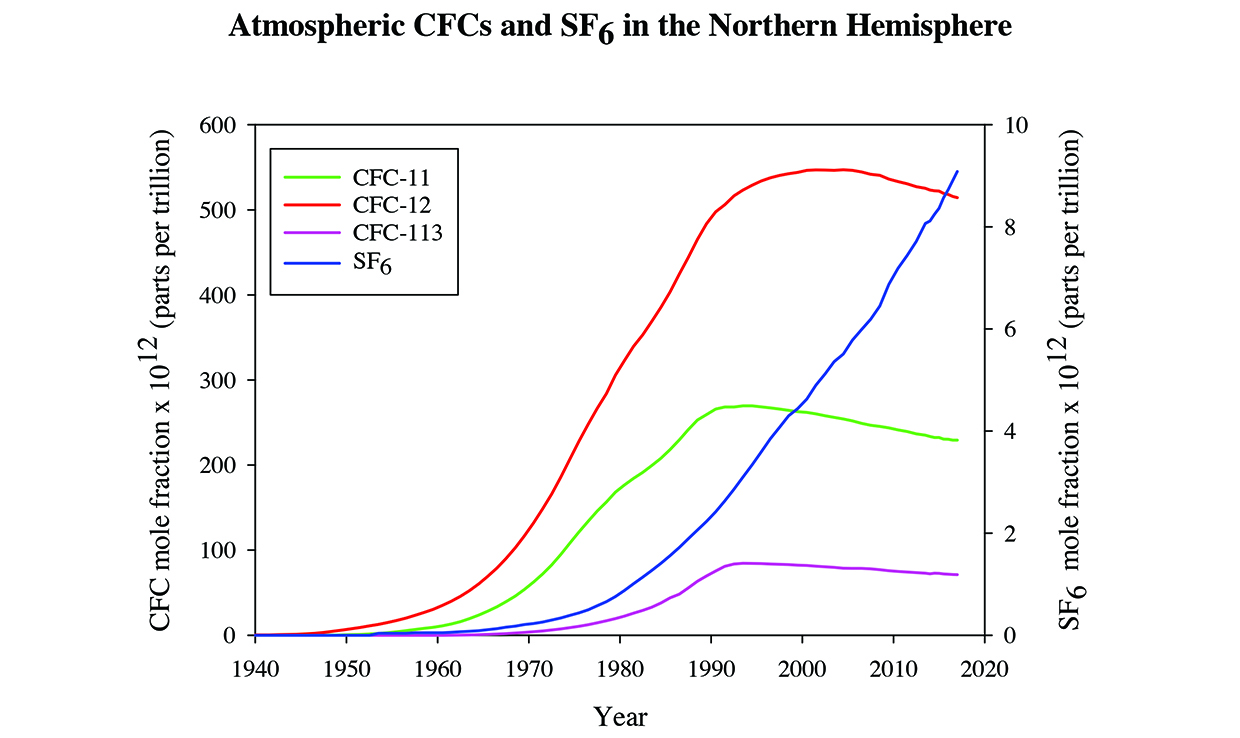
CFCs are potentially useful tracers of groundwater flow because they are non-reactive and their input history is well known. CFC-12 (CCl2F2) and CFC-11 (CCl3F) were introduced into the atmosphere in the late 1940’s while CFC-113 (CCl2FCClF2) was first released to the atmosphere in the 1960’s. The atmospheric concentrations of these CFCs increased until 1993 for CFC-113, 1994 for CFC-11, and 2004 for CFC-12 and decreased since the maximum concentrations were reached. The recent decreasing CFC concentrations have complicated their use as transient tracers because in some cases two ages are possible. SF6 was first introduced into the atmosphere in the 1950’s and has been increasing in concentration to the present. Therefore by measuring the CFCs and SF6 a distinct recharge age for groundwater can be determined for samples ranging from 0 to 70 years old.
When water is in contact with the atmosphere (i.e. during recharge) it picks up a CFC or SF6 signature based on the atmospheric concentration and the temperature dependent solubility coefficient of each compound. When isolated from the atmosphere the groundwater retains its characteristic CFC and SF6 concentration because the compounds are non-reactive (conservative) under aerobic conditions, and relatively little mixing occurs in ground water systems (compared to the ocean). The CFC and SF6 tracers offer several advantages to the more commonly used tritium/helium-3 tracer pair. A CFC/SF6 analysis is less costly than the tritium/helium-3 analysis. CFCs also offer the advantage of essentially real-time data. The typical turnaround time is 1 week, while samples for tritium/helium-3 analysis can take from several weeks to several months for complete analysis. Offsetting some of these advantages are the stringent sampling requirements needed to obtain valid CFC and SF6-derived recharge ages. For addition information on sampling groundwater for CFC/SF6 tracer analysis go to: Advice on CFC/SF6 Sampling.
Water samples are analyzed for CFCs and SF6 using a custom built purge-and-trap gas chromatograph with electron capture detection. Briefly, water samples are purged with inert gas to remove dissolved gases.
The CFCs and SF6 are adsorbed from the purging gas stream onto trap held at -20o C. Once the CFCs and SF6 are purged and trapped, the trap is heated to release the CFCs and SF6 onto a small volume-focusing trap held at -20o C. When CFC and SF6 transfer is complete, the focusing trap is heated releasing the CFCs and SF6 into the gas chromatograph Separation of the CFCs and SF6 is achieved on a capillary column and the compounds are detected using an electron capture detector. This method is extremely sensitive and the limit of detection for the CFCs are 0.005 x10-12 moles per kilogram of water (pmol kg-1). The limit of detection for SF6 is even more sensitive at 0.05 x 10-15 moles per kilogram of water (fmol kg-1). Precision values for all three CFCs are 2 % or less. SF6 precision is 5% or less. The accuracy of CFC and SF6-derived recharge ages from these measurements is 2 years or less.





